|
Drug that has sickened dozens banned
(Xinhua)
Updated: 2006-08-04 12:21 The Chinese Ministry of
Health issued an urgent circular ordering the disuse of a problematic injection
medicine used to treat bacteria infections, which probably has caused at least
one death.
A six-year-old girl from Harbin, the capital of northeast
China's Heilongjiang Province, was reportedly killed from having been mainlined
with this problematic antibiotics known as clindamycin phosphate glucose
injection.
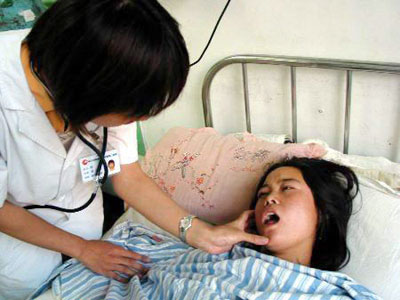
A doctor checks a
patient suffering an adverse response to an injection of Xin Fu at the
Harbin Medical University's Second Affiliated Hostipal August 3, 2006.
[Xinhua] |
An
increasing number of patients from provinces and regions including Qinghai,
Guangxi, Zhejiang, Heilongjiang and Shandong have complained about symptoms
ranging from chest distress, pain in the kidney of the body, bellyache,
diarrhea, nausea, vomit, to anaphylactic shock after having been injected with
the clindamycin phosphate glucose injections produced by the Anhui company.
The dead, identified as Liu Sichen, had an intravenous injection of the
clindamycin phosphate at about 2 p.m. on July 27 for common cold, but she
developed symptoms including high fever within 20 minutes, according to Sun
Pengli, director of the Adverse Drug Reaction (ADR) Monitoring Center of Harbin
City, on Friday.
The clindamycin phosphate glucose injection mainlined
into the girl patient was known to be produced by Anhui Huayuan Worldbest
Biology Pharmacy Co., a subsidiary of Shanghai Worldbest Co.Ltd., with a batch
number of 06062602.
The patient was soon transferred to the State Farm
General Hospital of Heilongjiang Province for further rescue operation. The girl
remained in a coma.
She was again transferred to the No.2 Hospital of
the Harbin Medical Sciences University, but was proclaimed dead at the night of
July 27 despite all rescue efforts by medical workers.
"Based on all
materials we have gathered, a preliminary judgement can be made that the girl
was killed due to the injection of the clindamycin phosphate glucose produced by
the Anhui Huayuan Worldbest Biology Pharmacy Co.," said Sun Pengli.
In
the meantime, sources from Penglai City of Shandong Province, and Heilongjiang
and Qinghai provinces said an upward of 21 people were sickened by the
intravenous injection of the clindamycin phosphate glucose, nine of whom are
still receiving ICU treatment at their respective local hospitals.
Food
and drug authorities in Shandong, Qinghai provinces have banned sale and use of
the problematic injections. And food and drug officials in Heilongjiaing
collected samples of the problematic injections and had them tested with the
provincial pharmaceuticals testing lab.
The Ministry of Health demanded
that all batches of clindamycin phosphate glucose injections produced in the
past two moths by Anhui Huayuan Worldbest Biology Pharmacy Co. be suspended from
use immediately.
Fake of bad drugs have killed dozens of people in China
in recent years and raised questions about drug safety.
The country has
recently fined Qiqihar No.2 Pharmaceutical Co. Ltd. and revoked its licence
after its drug meant to treat gastric disorders killed 11 people and turned out
to be bogus.
Anhui Huayuan Worldbest Biology Pharmacy Co., has been told
by the Anhui Provincial Food and Drug Bureau to halt production at the injection
workshop and to cooperate with the special groups in the investigative effort.
According to a spokesman for the provincial food and drug bureau,
several groups have stationed at the Anhui Huayuan Worldbest Biology Pharmacy
Co., doing investigations there.
"The production can not be resumed
until a clear outcome is out about the fuss," said the spokesman, who added the
bureau ordered a province-wide inspection over all enterprises for producing
large-capacity injections.
A recall of the problematic drug is also
underway.
While ordering that an inventory be made into the stockpile of
the injections and all unused injections be sealed properly, the ministry
circular also asked medical and health organizations not to purchase the
injections made by the above-mentioned company.
In the meantime, the
circular also told medical organizations to arrange medical workers to closely
monitor patients who have had the injections and go all out to rescue those
patients who have shown serious clinical symptoms.
Clindamycin phosphate
glucose injections are mainly used to treat bacteria infections caused by
gram-positive bacterium and gram-positive anaerobic bacterium. Stated side
effects are mainly restricted to the gastrointestinal tract and anaphylactic
reaction, sometimes coupled with abnormalities with the the liver and kidney
body parts.
|
| |
|
| |
|
|
|
| Most Commented/Read Stories in 48 Hours |
|
|
|
|