Zhifei Lvzhu vaccines gain GMP recognition
( Etimes )
The combined vaccine of AC meningococcus (combined) and haemophilus influenzae type B (combined) (hereinafter referred to as "AC-Hib vaccine") and A + C meningococcus polysaccharide conjugate vaccine (hereinafter referred to as "AC conjugate vaccine") independently developed by Zhifei Lvzhu Biopharmaceutical have passed site inspection and review and have been recognized to conform to the Good Manufacturing Practice for Pharmaceutical Products (Amended in 2010). The AC-Hib vaccine is exclusive to Zhifei Lvzhu Biopharmaceutical and has not been registered or produced in China.
It is reported that the above two vaccines are now in the publicity stage, which will last 10 workdays until Sept 12. After the publicity stage, the GMP certificate can be issued if there is no objection. Zhifei Lvzhu Biopharmaceutical prompts that even after the GMP certificate is issued, the products cannot be sold in the market before batch release. The batch release may take three to four months so the final time for products sold in the market is uncertain. As calculated, the time for the above two vaccines sold in the market may be at the end of this year or the beginning of next year.
It is known that the AC-Hib vaccine is used to prevent meningitis, pneumonia, blood poisoning, phlegmon, arthritis, epiglottitis and other infectious diseases caused by the A + C meningococcal germ and haemophilus influenzae type B.
It can replace the AC meningococcal vaccine and Hib vaccine, reduce injection times, benefit the arrangement of vaccination procedures and decrease the pain from several vaccinations. The product is applicable to children who are two-months old and older. A complete immune procedure requires four vaccinations.
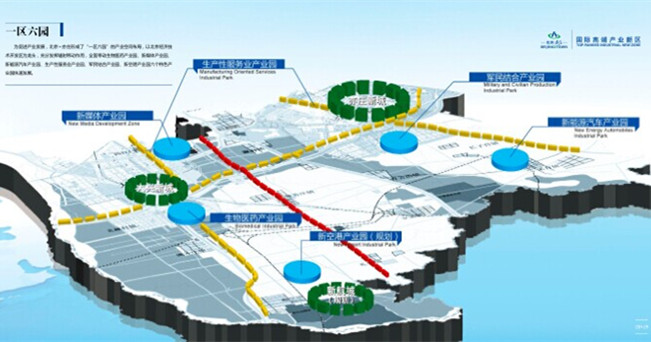 The Area with Six Parks
The Area with Six Parks Global Top 500
Global Top 500