Fighting cancer with genetics
Updated: 2012-07-29 07:13
By Gina Kolata(The New York Times)
|
|||||||||
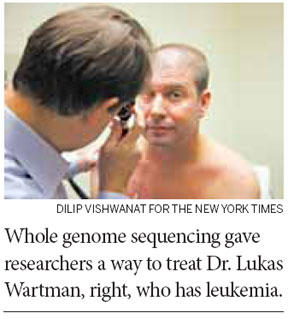
St. Louis - Genetics researchers here at Washington University, one of the world's leading centers for work on the human genome, were devastated. Dr. Lukas Wartman, a young, talented and beloved colleague, had the very cancer he had devoted his career to studying. So one day last July, Dr. Timothy Ley, associate director of the university's genome institute, summoned his team. Why not dedicate everything we can to see if we can find a rogue gene spurring Dr. Wartman's cancer, adult acute lymphoblastic leukemia, he asked? "It's now or never," he recalled telling them. "We will only get one shot."
Dr. Ley's team tried a type of analysis that they had never done before. They fully sequenced the genes of both his cancer cells and healthy cells for comparison, and at the same time analyzed his RNA, a close chemical cousin to DNA, for clues to what his genes were doing.
The researchers on the project put other work aside for weeks, running one of the university's 26 sequencing machines and supercomputer around the clock. And they found a culprit - a normal gene that was in overdrive, churning out huge amounts of a protein that appeared to be spurring the cancer's growth.
Even better, there was a promising new drug that might shut down the malfunctioning gene - a drug that had been tested and approved only for advanced kidney cancer. Dr. Wartman became the first person ever to take it for leukemia. And now his cancer is in remission and has been since last fall.
While no one can say that Dr. Wartman is cured, after facing certain death last fall, he is alive and doing well. Dr. Wartman is a pioneer in a new approach to stopping cancer. What is important, medical researchers say, is the genes that drive a cancer, not the tissue or organ where the cancer originates.
One woman's breast cancer may have different genetic drivers from another woman's and, in fact, may have more in common with prostate cancer in a man or another patient's lung cancer.
Under this new approach, researchers expect that treatment will be tailored to an individual tumor's mutations, with drugs that hit several key aberrant genes at once. The cocktails of medicine would be analogous to H.I.V. treatment, which uses several different drugs at once.
Researchers differ about how soon the method, known as whole genome sequencing, will be generally available and paid for by insurance. But they believe that it has enormous promise.
"Until you know what is driving a patient's cancer, you really don't have any chance of getting it right," Dr. Ley said. "For the past 40 years, we have been sending generals into battle without a map of the battlefield. What we are doing now is building the map."
For now, whole genome sequencing is in its infancy and dauntingly complex. The gene sequences are only the start. The arduous job is to figure out which mutations are important.
So far, most who have chosen this path are wealthy and well connected. When Steve Jobs had exhausted other options to combat pancreatic cancer, he consulted doctors who coordinated his genetic sequencing and analysis. It cost him $100,000, according to his biographer.
Dr. Wartman was included by his colleagues in a research study, and his genetic analysis was paid for by the university and research grants. Such opportunities are not available to most patients, but Dr. Ley noted that no patients were being neglected because of the urgent work to figure out Dr. Wartman's cancer.
A Life in Medicine
In college, Dr. Wartman was drawn to research on genetic changes that occur in cancers of the blood. In the winter of 2002, when he went to California to be interviewed for a residency program at Stanford University, he was nearly paralyzed by an overwhelming fatigue.
He rallied enough to get through the day. When he returned to St. Louis, he gave up running, too exhausted for the sport he loved. He started having night sweats. "I thought it might be mono," he said. "And I thought I would ride it out."
But then the bones in his legs began to hurt. He was having fevers. He went to an urgent care center in February 2003. The doctor noticed that his red and white blood cell counts were low. And Lukas Wartman, who had been fascinated by the biology of leukemia, began to suspect he had it.
The next day, he went back for more tests. A doctor slid a long needle into his hip bone and drew out marrow for analysis.
"We looked at the slide together," Dr. Wartman said. "It was packed with leukemia cells. I was in a state of shock."
Dr. Wartman underwent nine months of intensive chemotherapy, followed by 15 months of maintenance chemotherapy. Five years passed when the cancer seemed to be gone. But then it came back. Next came intensive chemotherapy, to put the cancer into remission, followed by a bone-marrow transplant from his younger brother.
Seven months after the transplant, he went to a major cancer meeting and sat in on a session on his type of leukemia. The speaker reported that only 4 or 5 percent of those who relapsed survived.
"My stomach turned," Dr. Wartman said. "I will never forget the shock of hearing that number."
By last spring, three years after his transplant, Dr. Wartman was running nine to eleven kilometers every other day and feeling good. "I thought maybe I would run a half marathon in the fall."
Then the cancer came back.
A Clue in RNA
Dr. Wartman's doctors realized then that their last best hope for saving him was to use all the genetic know-how and technology at their disposal.
After their month of frantic work to beat cancer's relentless clock, the group had the data.
The cancer's DNA had many mutations, but there was nothing to be done about them. There were no drugs to attack them.
But the other analysis, of the cancer's RNA, showed that a normal gene, FLT3, was wildly active in the leukemia cells. Its normal role is to make cells grow and proliferate. An overactive FLT3 gene might be making Dr. Wartman's cancer cells multiply so quickly.
Even better, there was a drug, sunitinib or Sutent, approved for treating advanced kidney cancer, that inhibits FLT3.
But it costs $330 a day, and Dr. Wartman's insurance company would not pay for it. He also pleaded with the drug's maker, Pfizer, to give him the drug under its compassionate use program, explaining that his entire salary was only enough to pay for
7 1/2 months of Sutent. But Pfizer turned him down too.
He gathered up the money to buy a week's worth and began taking it. Within days, his blood counts were looking more normal.But he said he was afraid to hope, and the cost of the drug nagged at him. If it worked, how long could he afford to keep taking it?
The next day, a nurse at the hospital pharmacy called with what seemed miraculous news: a month's supply of Sutent was waiting for Dr. Wartman. The doctors in his division had pitched in to buy the drug.
Two weeks later, his bone marrow, which had been full of leukemia cells, was clean, a biopsy showed. Two more sensitive tests confirmed the cancer's remission. "I can't believe it," his physician, Dr. John DiPersio, told him.
Hunches and Decisions
Dr. Wartman's doctors considered whether he should keep taking Sutent or have another bone-marrow transplant now that he was in remission again.
In the end, Dr. DiPersio decided Dr. Wartman should have the transplant because without it the cancer might mutate and escape the Sutent. Meanwhile, Pfizer had decided to give him the drug.
Dr. Wartman's cancer is gone, for now, but he has struggled with a common complication of bone-marrow transplants, in which the white blood cells of the transplanted marrow attack his cells as though they were foreign. He has had rashes and felt ill. But these complications are lessening, and he is back at work in Dr. Ley's lab.
His colleagues want to look for the same mutation in the cancer cells of other patients with his cancer. And they would like to start a clinical trial testing Sutent to discover whether the drug can help others with leukemia, or whether the solution they found was unique to Lukas Wartman.
Dr. Wartman himself is left with nagging uncertainties. He knows how lucky he is, but what does the future hold? Can he plan a life? Is he cured?
"It's a hard feeling to describe," he said. "I am in uncharted waters."
The New York Times
(China Daily 07/29/2012 page11)