Economy
"Zero tolerance" for quality problems of essential drugs
Updated: 2011-01-14 13:22
(Xinhua)
BEIJING -- An official with China's top drug regulator has vowed "zero tolerance" for any problems that may undermine the quality of more than 300 essential pharmaceuticals covered by a nationwide system.
Shao Mingli, head of the State Food and Drug Administration (SFDA), made the call Thursday at a national meeting on the supervision and management of drugs and food.
All pharmaceuticals under the system will be monitored electronically by the end of 2011, the SFDA said in a statement at the meeting.
China began implementing the essential medicine system in 2009 to reduce costs for patients.
The system will cover most government-sponsored health institutions at the grass roots by the end of the year and drugs sold there will have zero markup.
Due to longstanding low government funding for state-run hospitals, which in many places only covers 10 percent of operating costs, doctors have often aggressively prescribed expensive, and sometimes unnecessary, medicines and treatment to generate income for the hospitals.
China is one of the world's largest markets for pharmaceuticals, with total industrial output value of the sector estimated at 1.2 trillion yuan (about $181.7 billion) for the year of 2010, according to the statistics released at the meeting.
Specials

President Hu visits the US
President Hu Jintao is on a state visit to the US from Jan 18 to 21.
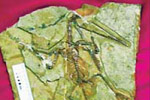
Ancient life
The discovery of the fossile of a female pterosaur nicknamed as Mrs T and her un-laid egg are shedding new light on ancient mysteries.

Economic Figures
China's GDP growth jumped 10.3 percent year-on-year in 2010, boosted by a faster-than-expected 9.8 percent expansion in the fourth quarter.



