'Human power plant': How the cell's molecular machines work
Every cell in our body is like a workshop full of machines. The machines are embedded in mitochondrial membranes – microscopic energy centers. They serve to synthesize ATP (adenosine triphosphate) – sort of a fuel for humans that our whole body uses in order to function properly.
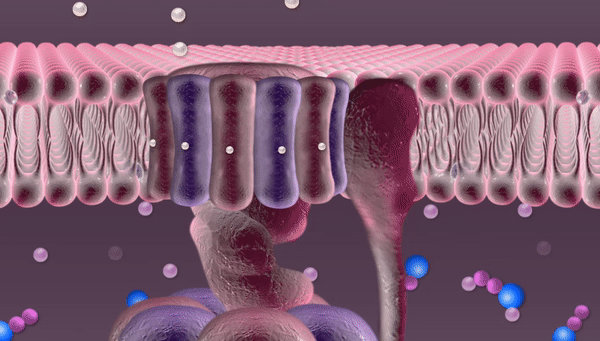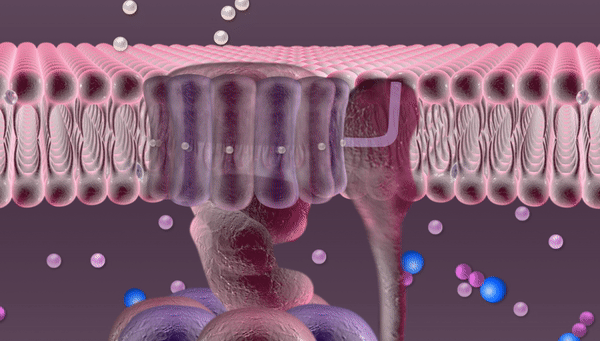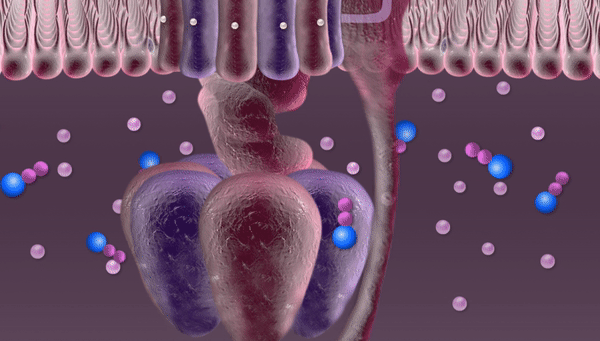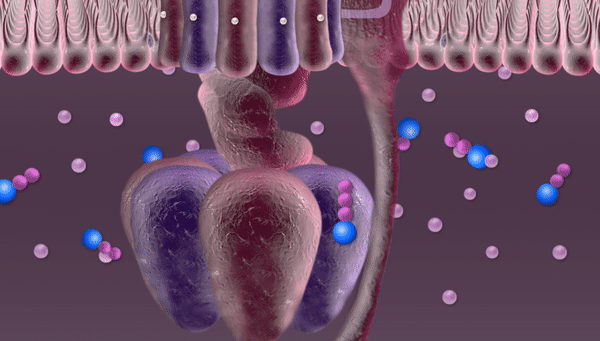
Every second hundreds of trillions of mitochondria are buzzing with the roar of robotic devices running on proton power. Once released through a tiny hole, phosphate falls into a container, acquires new chemical properties through the electric current of protons and is then released through a pipe into the mitochondrion to join other phosphates in forming an ATP molecule.
How can this industrial process of sorts really be taking place in our body? Where do these "machines" in our cells come from? Where do the protons come from? Why do millions of these devices reminiscent of revolving doors inhabit our bodies?
These questions, and the answers to them, came from microbiologists and biophysicists who wanted to find out exactly how food actually provides every muscle in the human body with energy. What they discovered is that the cycle of transformations is so complex that a detailed explanation of this process could take up a large book. In short, it can be described like this: once you chew and swallow your food, it goes to your stomach, where it undergoes various transformations that allow for further absorption. The digestion process continues in the small intestine with the help of various enzymes. That's where the transformation of carbohydrates into glucose and the breakdown of lipids and proteins take place.
Then glucose enters the cell. In the cell it breaks down in two parts, and at this stage (during which it is called pyruvate) it moves on to the mitochondrion.
Upon entering the mitochondrion, pyruvates — products of glucose in the Krebs cycle — oxidize.
The mitochondrion also contains nicotinamide adenine dinucleotide (NAD). The energy released by oxidation detaches a proton from NAD.
This proton is the goal of this whole complex series of transformations. Protons are necessary for synthesizing ATP molecules. In our illustration these protons are depicted rushing over the mitochondrial membrane before entering the "machine". In fact, until recently no one really knew how they get there, since these protons are free to float away wherever they like. But for some reason they remain close to the membrane, crowding right in front of the revolving doors of the "machine". Russian researchers at the National University of Science and Technology MISIS (NUST MISIS) and their colleagues from the Institute of Biophysics at Johannes Kepler University (Linz) have conducted complex experiments and now know why this happens.
"While moving about inside the mitochondrion, protons are surrounded with water," explained Sergei Akimov, research fellow at the NUST MISIS Department of Theoretical Physics and Quantum Technologies. "We know that a molecule of water is made up of two hydrogen (H1) atoms and one oxygen (O16) atom. Apart from forming chemical bonds within a molecule of water, these atoms can also form weak bonds with neighboring molecules called hydrogen bonds. Near the membrane surface these bonds in the molecules of water are formed in a different way, since there is water on one side and the ‘wall' on the other side. Hydrogen bonds formed near the membrane surface are different; they have a different bond number, a different structure. And protons use these bonds as "tracks" to move along the membrane. Our research proved that protons 'like' this structure, they don't move towards the center of the mitochondrion, but rush along the membrane at abnormally high speeds."
The results of this fundamental research bring scientists closer to understanding the global mechanisms of energy generation in cells, and open up new possibilities in pharmacology. The results of the research can be used to develop drugs that will neutralize the effects of uncoupling poisons and prevent hyperthyroid diseases. In bodies with these pathologies, mitochondria accumulate uncoupling agents — weak lipid soluble acids that effectively bind protons together, leading to an overall decrease in ATP synthesis. The new information acquired by Russian scientists will allow us to get closer to understanding what has to be done in order to replenish our energy at the level of every cell.