WHO assesses vaccines from China
By CHEN WEIHUA in Brussels | chinadaily.com.cn | Updated: 2021-01-23 04:15
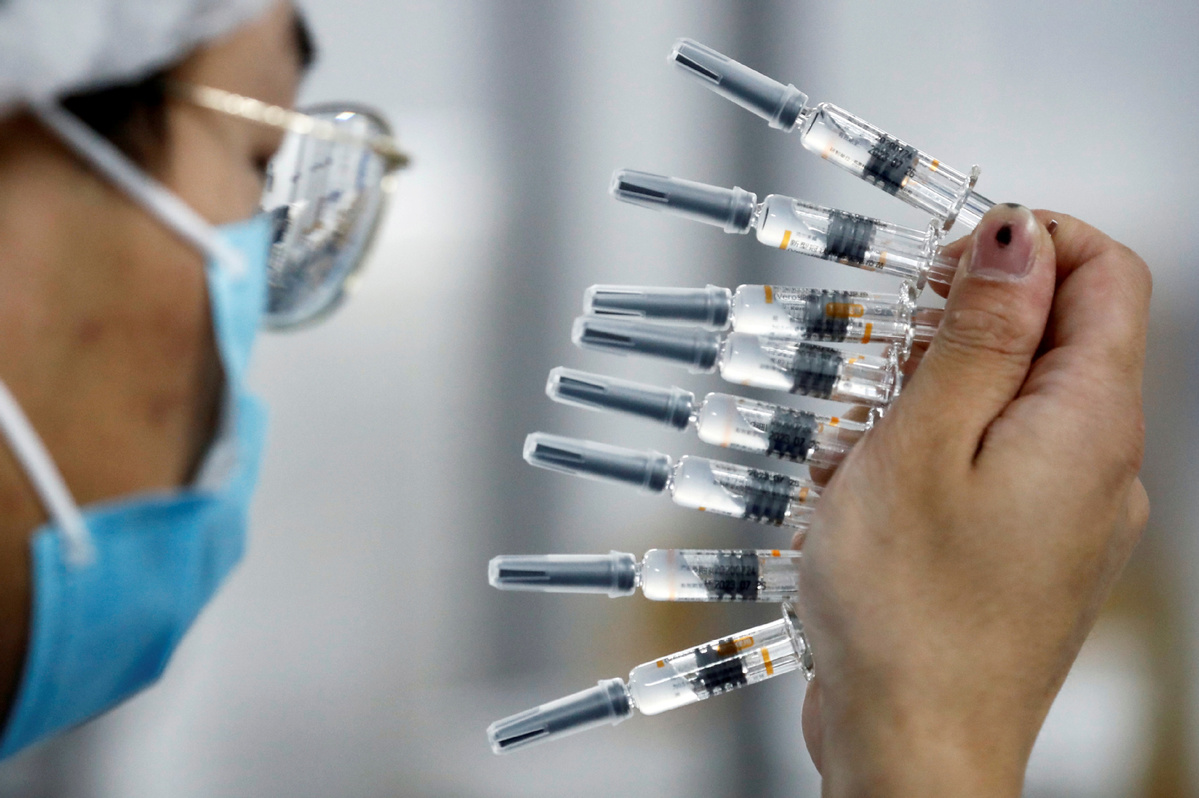
The World Health Organization, or WHO, said on Friday it is assessing vaccines from Chinese producers for possible emergency use in the COVAX global vaccine program.
Mariangela Simao, the WHO's assistant-director general for Access to Medicine and Health Products, said Chinese vaccine producers Sinopharm and Sinovac have sent full doses to the WHO that will be inspected next week.
"They are being assessed right now," Simao told a virtual press conference, in response to a question about the prospect of Chinese and Russian vaccines being included in the COVAX facility.
Seth Berkley, CEO of Gavi, the Vaccine Alliance, also confirmed that the vaccines are being considered.
He said: "We will consider any vaccines for inclusion in COVAX, assuming they add value to the portfolio, assuming that there is transparent data on safety and efficacy, and that we can come up with a reasonable price point and supply allocation that makes sense," he told the press briefing.
On Wednesday, Chinese Foreign Ministry spokeswoman Hua Chunying said that Chinese COVID-19 vaccine developers Sinovac, Sinopharm and CanSinoBio have submitted applications to COVAX.
She said Chinese companies are actively discussing cooperation with COVAX, which China joined some months ago.
"China is willing to contribute to the realization of vaccine accessibility and affordability in developing countries through COVAX," Hua said.
The Xinhua News Agency reported on Thursday that Sinovac is ramping up the production of CoronaVac, an inactivated COVID-19 vaccine (that uses killed versions of the virus), to ensure global supply.
The Sinovac vaccine has been approved for emergency use in several countries, including China, Indonesia, Brazil and Chile, according to Yin Weidong, chairman and CEO of the company.
"Sinovac has received vaccine orders from Brazil, Indonesia, Turkey, Chile and other countries and regions, and we are making every effort to expand the production capacity," said Yin.
On Friday, COVAX announced the signing of an advance purchase agreement for up to 40 million doses of the Pfizer-BioNTech vaccine. Additionally, it said that pending WHO emergency use listings, nearly 150 million doses of the AstraZeneca/Oxford candidate are anticipated to be available in the first quarter of this year.
"COVAX is therefore on track to deliver at least 2 billion doses by the end of the year, including at least 1.3 billion doses to 92 lower income economies in the Gavi COVAX AMC," it said. Th Gavi COVAX AMC is the initiative to supply vaccines to the 92 economies.
"Together, these announcements mean COVAX could begin delivering doses in February, provided we can finalize a supply agreement for the Pfizer/BioNTech vaccine, and emergency use listing for the AstraZeneca/Oxford vaccine," WHO Director-General Tedros Adhanom Ghebreyesus told the Friday briefing.
Tedros said he welcomes the United States' commitment to support the ACT Accelerator and to join COVAX. The ACT Accelerator is a WHO-led global initiative to develop, produce and equitably distribute COVID-19 tests, drugs and vaccines.
The US announced on Thursday that it plans to retain its membership in the WHO, after former US President Donald Trump formally began the process of withdrawing the US from the organization last July.