Splitting water with sunlight produces hydrogen

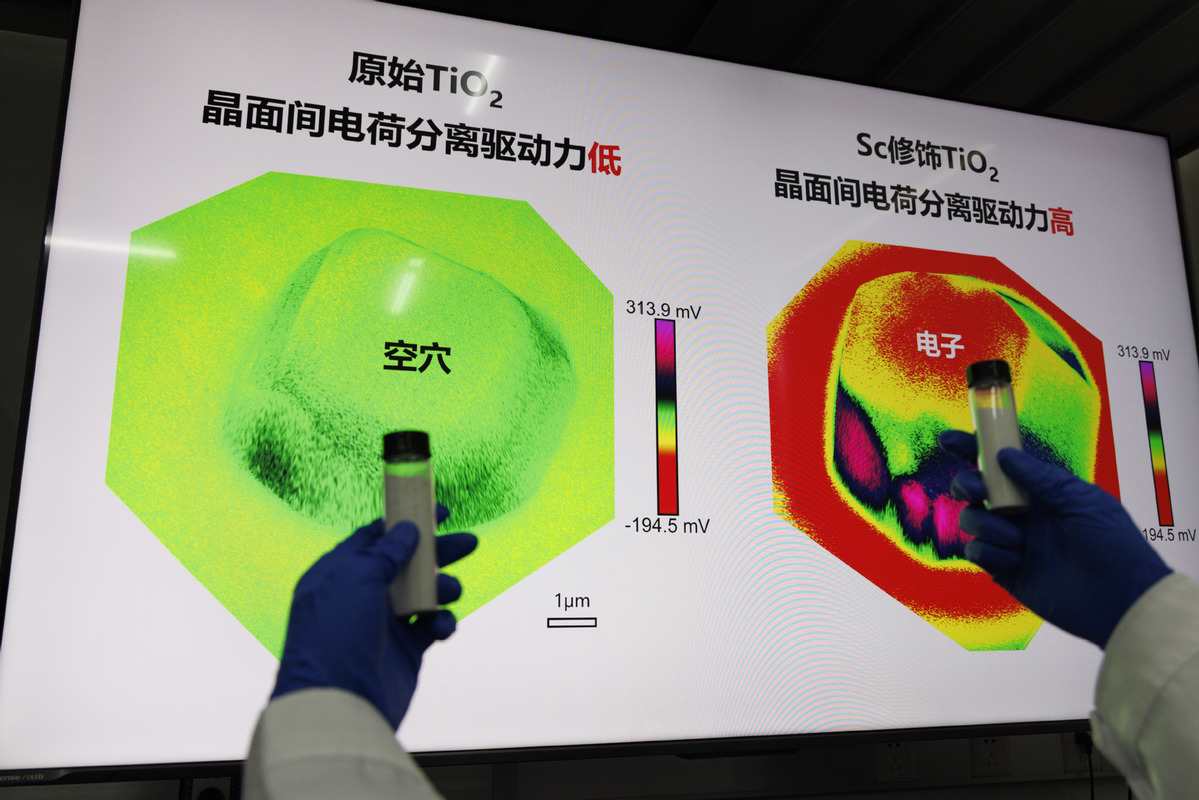
The key to directly splitting water with sunlight lies in a material called titanium dioxide. When exposed to sunlight, it functions like a microscopic power plant, generating energized electron-hole pairs that break down water molecules into hydrogen and oxygen, Liu explained.
However, traditional titanium dioxide has a critical flaw — its internal structure resembles a maze, causing the activated electrons and holes to collide randomly and recombine and annihilate within a millionth of a second. Additionally, the high-temperature fabrication process of the material often leads to oxygen atom loss, creating positively charged "trap zones" that capture electrons.
Liu's team addressed these issues by introducing scandium, a rare-earth element neighboring titanium on the periodic table, to restructure the material.
Scandium ions fit perfectly into the titanium dioxide lattice without causing structural distortion. Their stable valence neutralizes the charge imbalance caused by oxygen vacancies, eliminating "trap zones". Moreover, scandium atoms reconstruct the crystal surface, creating specific facets that act like "electronic highways and overpasses", allowing electrons and holes to escape the maze efficiently.