Stem cells offer ray of hope for healthy future
By Alfred Romann in Seoul For China Daily (China Daily) Updated: 2014-06-23 07:08"The institutional investor base in Asia is not very comfortable with biotechnology," says Nam Chul Park, head of Asia-Pacific healthcare research with HSBC. He explains that in South Korean companies, institutional investors account for about 2 percent, a figure that he believes should be at least 20 percent.
"(South) Korean biotech has had a lot of government support and government support is important," Park says.
Globally, the top 15 regenerative medicine products were worth an estimated $1 billion last year, four times more than in 2006. All but one of them are for skin, wound, bone or cartilage repair, like Medipost's product.
In the United States, the first regenerative medicine product hit the market in 1998. By 2011, they had been used to treat 500,000 patients.
But even as the science gets better and more interesting - even making it possible to tackle illnesses like Alzheimer's disease - investors remain wary.
"Investors don't understand the space. They think the cost of goods is too high," says Robert Preti, president and chief scientific officer at PCT Co Ltd, a US company that provides cell therapy services.
Preti is also a member of the executive committee of the Alliance for Regenerative Medicine, which lobbies for reform and tracks the space globally.
And the costs can be high. There are only a handful of regenerative medicine products on the market today. South Korea, which has taken a lead, has just three on the market.
Companies in this area spend years researching drugs, getting them through regulatory approvals and trying to get them to market. All this time, they have not a single bit of revenue coming in.
This is not uncommon in drug development. On average, just one in 10 drugs under development ever makes it to market. The cost of developing a single drug, including putting it through the various trials, could be upward of $1 billion.
To date, only a handful of countries have some kind of funding or regulatory framework for regenerative medicine and Japan, South Korea and Australia are among them.
South Korea, as most other places in Asia, is looking for ways to deal with the rising cost of healthcare. And the biologic drugs that facilitate regenerative medicines, such as stem cell products, have great promise. Biologic drugs are made from biological sources, as opposed to chemically synthesized products.
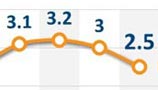
South Korea spent 7.4 percent of its GDP in 2012 on healthcare, up from 7.1 percent a year earlier and 5 percent a decade ago.
|
 |
 |
| Stem Cell Industrial Park under construction in Wenjiang | Medical experts call for effective stem cell treatment regulation |
- Tudou found guilty of violating CCTV copyright
- Fosun International to invest in Studio 8
- Zhengzhou Nissan recalls defective SUVs
- Banks raise record $14b offshore
- Guangdong sells local govt bonds
- Chinese official pledges decisive role for market
- Gasoline, diesel prices hiked
- CNOOC, private firm ink gas station deal