Biosimilars provide new growth options
By Alfred Romann (China Daily) Updated: 2014-06-23 07:08One ongoing concern for people and governments in Asia is the rising cost of healthcare. Every year, governments spend more on caring for their population while the people themselves worry that treatments and drugs could prove to be too expensive.
Since the 1990s, one way to lower costs has been to use generic drugs, which have made some of the most sought-after and expensive medicines in the world much cheaper.
But doing the same with the more complex biologic drugs that are often seen as the future of medicine may not be so easy.
Nevertheless, companies are betting heavily on the market for biosimilars, and health authorities are, slowly, making this possible. Across Asia, half a dozen countries already have active markets and more are on the way.
In the United States, generics brought down the price of drugs like the antidepressant Prozac from $40 per month to $10 per month. Over the last decade, the savings to patients from the use of generic drugs have risen three and a half times.
For emerging markets like much of Asia, the impact of generics on the pockets of patients has been even greater, particularly for complex and expensive chemical drugs like the antiretrovirals used to treat HIV/AIDS.
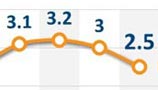
In 2007, a one-pill daily dose of the ARV combination tenofovir and lamivudine cost $613 per year in low-income countries and $1,033 in lower middle-income ones. The price has fallen 67 percent since then.
At the end of 2012, some governments negotiated a price of a little more than $100 for a two-pill combination of the same drugs, according to humanitarian organization Medecins Sans Frontieres, which tracks prices.
Generics are copies of chemical drugs that can be produced and sold once the patents on the original products run out. Patents last around 20 years, depending on which country grants them. A number of companies, most notably in India, emerged and expanded globally by producing and distributing generics.
Because chemical drugs are essentially mixtures of chemicals made in factories, the manufacturing process is relatively easy to copy to create a generic version. Now companies are trying to do the same with the biologic drugs that have emerged, over the last couple of decades, as the new wave of medicines.
|
 |
 |
| Stem Cell Industrial Park under construction in Wenjiang | Medical experts call for effective stem cell treatment regulation |
- Tudou found guilty of violating CCTV copyright
- Fosun International to invest in Studio 8
- Zhengzhou Nissan recalls defective SUVs
- Banks raise record $14b offshore
- Guangdong sells local govt bonds
- Chinese official pledges decisive role for market
- Gasoline, diesel prices hiked
- CNOOC, private firm ink gas station deal