WHO approval of Sinopharm vaccine set to benefit world coronavirus fight
By BO LEUNG in London | CHINA DAILY | Updated: 2021-05-10 07:02
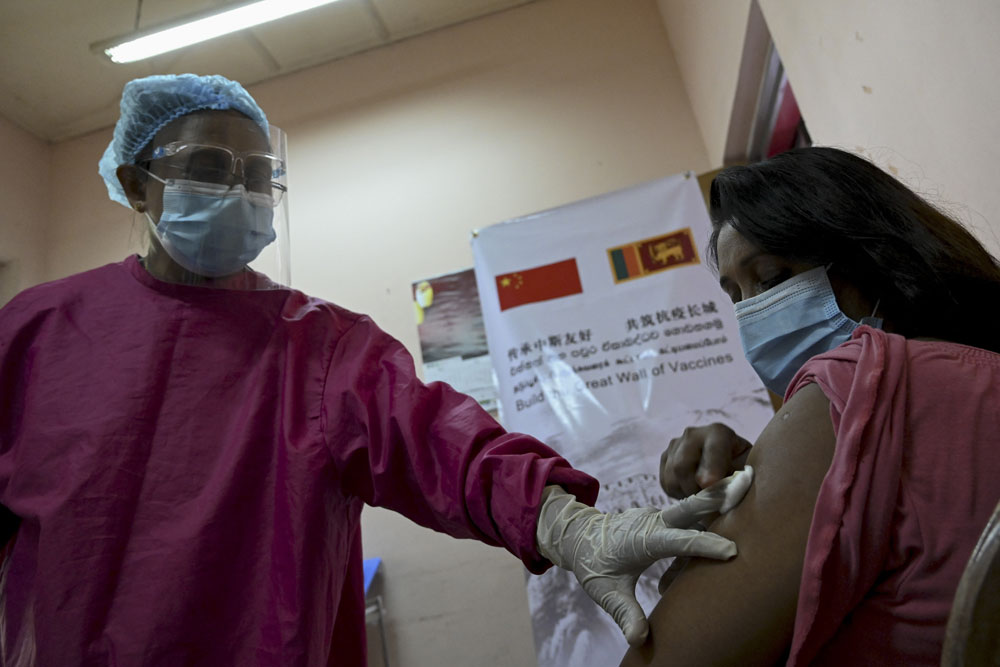
The World Health Organization's approval to give emergency use to the COVID-19 vaccine manufactured by China's Sinopharm has been welcomed by experts and health regulators worldwide.
On Friday, the WHO gave the vaccine the green light, paving the way for potentially millions of doses to be rolled out globally to reach countries in need and boost WHO-backed efforts such as the COVAX initiative.
COVAX is a global effort aimed at ensuring access in poorer nations to novel coronavirus vaccines.
The WHO is also considering approval for the emergency use of another Chinese vaccine made by Sinovac.
Andrea Taylor, an expert on global vaccine data at the Duke Global Health Institute, said two Chinese vaccines, if the Sinovac shot is included in the COVAX program, will constitute a "game changer".
"The situation right now is just so desperate for low- and lower-middle-income countries that any doses we can get out are worth mobilizing," Taylor told The New York Times. "Having potentially two options coming from China could really change the landscape of what's possible over the next few months."
Bangladesh is very pleased to learn about the WHO decision to include the Sinopharm vaccine in the emergency use listing, which surely comes as a big blessing for the whole world in the fight against the deadly disease, Mushtuq Hossain, an adviser at the Bangladesh Health Ministry's Institute of Epidemiology, Disease Control and Research, said in an interview with Xinhua News Agency.
"Our government has already signed an agreement with Chinese counterparts to import this vaccine," the health expert said.
Bangladesh's drug regulator has already approved the Sinopharm vaccine, Hossain said.
Speaking at the conference with South Asian foreign ministers that China hosted recently, he said that this was a good initiative by the Chinese government.
"International cooperation is a must for fighting a global pandemic like COVID-19," he said, noting that a WHO emergency use listing will help China extend more support to the countries in need.
Gavi, an international vaccine alliance organization that co-runs COVAX, welcomed the WHO's decision to approve the emergency use of the Chinese vaccine.
"This means the world has yet another safe and effective tool in the fight against this pandemic," the alliance was quoted by The Associated Press as saying.
The COVAX program has already distributed over 54 million doses of COVID-19 vaccines.
The WHO's move on Friday marks the first time any Chinese-made vaccine has received emergency authorization from the WHO.
During a media briefing, Tedros Adhanom Ghebreyesus, head of the WHO, said, "The WHO gave emergency use listing to Sinopharm Beijing's COVID-19 vaccine, making it the sixth vaccine to receive WHO validation for safety, efficacy and quality."
"This expands the list of COVID-19 vaccines that COVAX can buy, and gives countries confidence to expedite their own regulatory approval, and to import and administer a vaccine," he added.
Sinopharm joins WHO-approved vaccines for emergency use developed by Pfizer, Moderna, Johnson & Johnson, the Serum Institute of India and AstraZeneca.
"The addition of this vaccine has the potential to rapidly accelerate COVID-19 vaccine access for countries seeking to protect health workers and populations at risk," said Mariangela Simao, WHO assistant director-general for access to health products. "We urge the manufacturer to participate in the COVAX facility and contribute to the goal of more equitable vaccine distribution."
The WHO has recommended the Sinopharm vaccine for people aged 18 to 59 years, in a two-dose schedule with a spacing of three to four weeks.
The organization said the vaccine efficacy for symptomatic and hospitalized cases was estimated to be 79 percent for all age groups combined.
Unlike some other vaccines, the Sinopharm vaccine is easy to store, making it suitable for low-resource settings.
The WHO said it is also the first vaccine that will carry a vaccine vial monitor, a small sticker on the vaccine vials that changes color if the vaccine is exposed to heat, letting health workers know whether the vaccine can be used safely.
The Sinopharm vaccine has already been authorized by many countries around the world, with some 65 million doses distributed.
Xin Zhiming in London contributed to this story.